Opinion Editorial: Chemotherapy, Endothelial Senescence, and the Road to Safer Cancer Treatments
The world of cancer treatment is full of tricky parts and tangled issues that often seem overwhelming. Recent studies have shown that chemotherapeutic agents not only target cancer cells but also inadvertently trigger senescence in healthy endothelial cells. This opinion piece aims to take a closer look at chemotherapy-induced endothelial cell senescence, the role of oxidative stress and mitochondrial dysfunction, and how specific interventions can help reduce unwanted side effects. We will dig into the scientific findings with a neutral tone, using everyday language to explain the fine points and nitty-gritty details involved in the process.
Even though the technical data might initially appear intimidating and full of problems, a closer examination reveals opportunities for mitigating these side effects. By better understanding the subtle parts of oxidative stress pathways, reactive oxygen species (ROS) generation, and the intricate roles of proteins like ATM, researchers can figure out a path to designing safer chemotherapeutic regimens that preserve endothelial function without compromising anti-cancer efficacy.
Taming the Dizzying World of Cellular Senescence
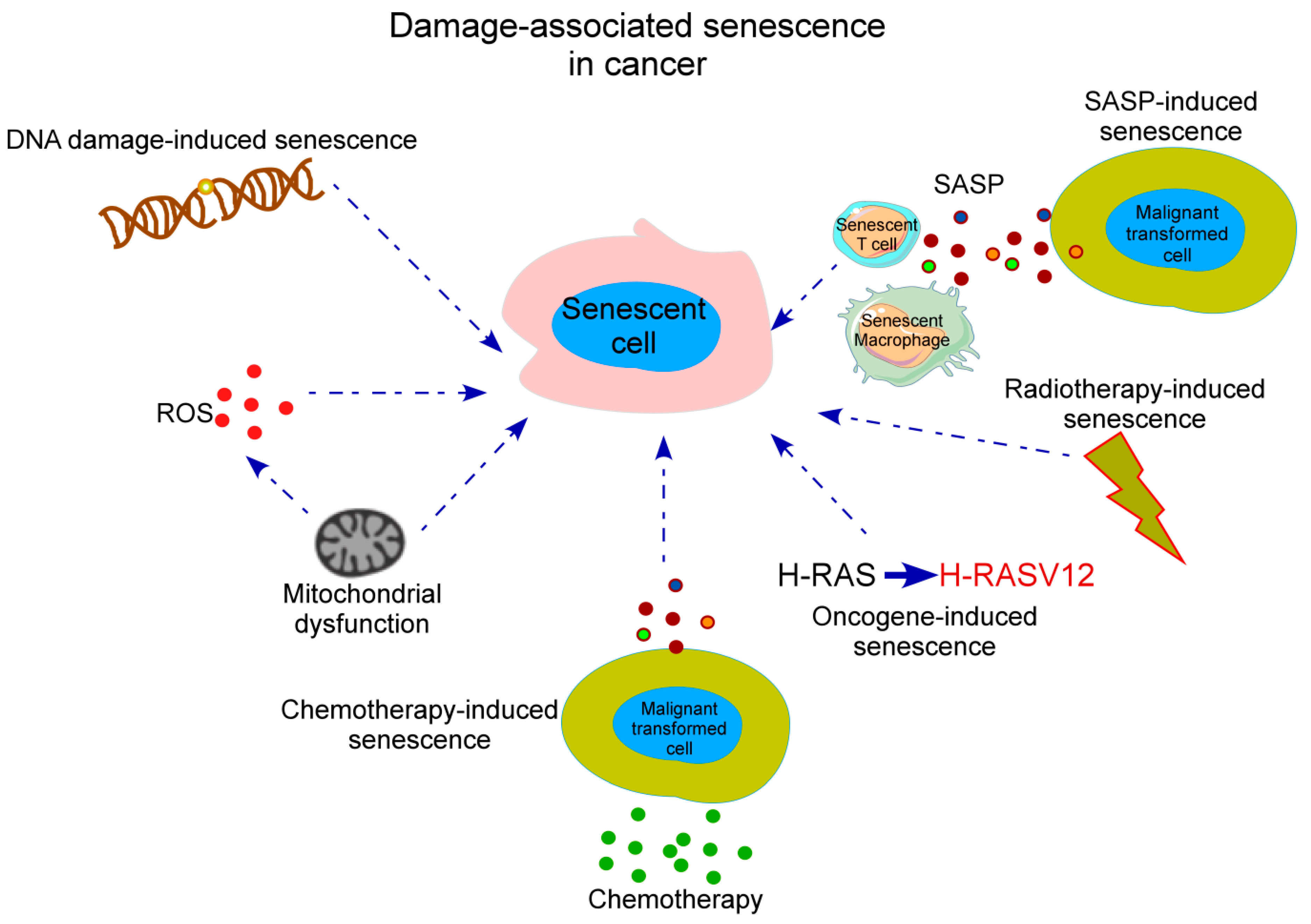
Cellular senescence is a state of irreversible cell cycle arrest where cells lose their ability to divide properly after being exposed to various stressors. In cancer therapy, the side effect of senescence in endothelial cells—the cells lining the blood vessels—can be particularly nerve-racking. When these cells enter a senescent state, they tend to secrete a cocktail of inflammatory elements known as the senescence-associated secretory phenotype (SASP). These secretions include cytokines and chemokines that contribute to chronic inflammation and can adversely affect tissue regeneration.
While senescence might initially slow down tumor growth by halting the proliferation of damaged cells, it can also promote a delicate imbalance that ultimately exacerbates tissue injury. The inflammatory mediators, when over-secreted, might create a microenvironment that is loaded with issues. As clinicians and researchers work together, it becomes critical to take a closer look at the fine details of how chemotherapy drugs provoke these changes in endothelial cells.
Unraveling the Twists and Turns of Chemotherapy’s Side Effects
Chemotherapy continues to be an essential tool in cancer treatment. However, the unwanted side effects on the vascular system are among the most intimidating and nerve-racking outcomes. Drugs such as doxorubicin, mitomycin C, etoposide, camptothecin, and methotrexate have proven effective in damaging cancer cells, but their effects on human umbilical vein endothelial cells (HUVECs) illustrate some complicated pieces of the puzzle in drug safety.
Many of these drugs work by damaging DNA, either through crosslinking or by inhibiting key enzymes such as topoisomerases. This damage not only quickly kills cancer cells but also leaves healthy cells with confusing bits of DNA damage that ultimately trigger senescence. The result is a spectrum of effects that vary from one chemotherapeutic agent to another, with some causing as high as 80% of cells to express senescence markers, while others trigger a more modest inflammatory response.
Below is a table summarizing some common drugs, their primary mechanisms, and the observed effects on endothelial cells:
| Drug | Primary Mechanism | Senescence Induction (%) | Key Observations |
|---|---|---|---|
| Doxorubicin (DOX) | DNA intercalation & topoisomerase II inhibition | High | Intense DNA damage markers and fragmentation |
| Mitomycin C (MMC) | DNA crosslinking | High | Strong DNA damage response and disrupted cytoskeleton |
| Etoposide (ETO) | Topoisomerase II inhibition | Up to 80% | Multifold effects with robust senescence features |
| Camptothecin (CPT) | Topoisomerase I inhibition | Moderate to high | Marked reduction in proliferative marker Ki67 |
| Methotrexate (MTX) | Dihydrofolate reductase inhibition | Lower (around 40%) | Prominent ROS generation leading to senescence |
This organized look at the data helps us understand the fine shades among different chemotherapeutic agents. It is clear that while each drug has a common goal of killing cancer cells, their collateral damage to endothelial cells is full of problems and loaded with potential long-term consequences.
Understanding ROS and Mitochondrial Dysfunction
One of the key drivers behind the senescence observed in endothelial cells is the disturbing surge of reactive oxygen species (ROS). While ROS are essential molecules that help in signaling and cell regulation, an imbalance can lead to oxidative stress—a condition where the fine balance of ROS production and clearance is tipped toward excessive amounts that damage cellular components.
In many chemotherapeutic treatments, elevated levels of ROS are associated with mitochondrial dysfunction. Mitochondria, known as the powerhouses of the cell, play a critical role in producing energy. When these organelles are compromised by drugs such as methotrexate or camptothecin, they start releasing excessive ROS, which in turn inflict further DNA damage and push cells to a state of senescence.
This cascade illustrates the complicated pieces involved in drug toxicity. To break it down, here are some key points:
- Oxidative Stress: ROS overproduction directly damages cellular lipids, proteins, and DNA.
- Mitochondrial Impairment: Drug-induced mitochondrial fragmentation disrupts energy production and worsens oxidative stress.
- DNA Damage: Increased ROS levels lead to the formation of markers like γH2AX and 53BP1, indicating double-strand DNA breaks.
- Cellular Senescence: The accumulation of oxidative damage drives cells to enter a senescent state, where their function is permanently altered.
Collectively, these factors contribute to the delicate balance of cellular aging within vascular tissue. Although cancer therapy requires strong interventions, the inadvertent activation of these pathways can result in a microenvironment that is on edge, complicating patient outcomes and long-term health.
Exploring the Role of ATM and ROS Scavengers
In the effort to protect healthy tissues, researchers have been investigating ways to interrupt the progression from DNA damage to cell senescence. Key players in this process include the protein ATM (ataxia telangiectasia mutated), which is activated by DNA double-strand breaks, and ROS scavengers like Mito-Q that help neutralize oxidative stress.
ATM has long been considered a master regulator of the DNA damage response. When chemotherapy drugs induce DNA lesions, ATM works to repair the damage by activating a cascade of molecular events. However, in many cases, the damage is so severe that even ATM’s efforts are not enough to reverse the effects, and the cell enters a senescent state as a protective measure. Studies have shown that while ATM inhibitors like KU55933 do reduce the activity of this protein, they fail to significantly alter the progression of senescence induced by drugs like doxorubicin, MMC, etoposide, or camptothecin. These observations suggest that once irreversible damage is set in motion, simply blocking ATM activity isn’t sufficient to reverse the negative outcomes.
On the other side, targeting the source of oxidative damage itself with mitochondrial antioxidants such as Mito-Q seems promising for certain drugs. Mito-Q, a mitochondria-targeted antioxidant, works by directly accumulating within mitochondrial membranes, thereby scavenging the surplus ROS. Notably, its protective effects are most pronounced in cases like methotrexate-induced senescence, where ROS generation plays a key role. Here, using Mito-Q has been observed to significantly alleviate markers of senescence and the expression of inflammatory cytokines such as IL-6 and IL-8.
It is important to note, however, that Mito-Q’s effectiveness varies. While it shows promise for methotrexate-treated cells, it does not offer the same benefits for drugs that cause extensive direct DNA damage like doxorubicin or etoposide. This difference highlights the need to appreciate the little twists in the mechanisms behind each drug’s side effects. When dealing with these diverse effects, one must figure a path tailored to each scenario rather than applying a one-size-fits-all approach.
Testing the Waters: Preclinical Observations and Their Implications
Preclinical studies using human umbilical vein endothelial cells (HUVECs) have provided a valuable window into the real-world implications of chemotherapy-induced vascular damage. In these experiments, methods such as senescence-associated β-galactosidase (SA-β-gal) staining, immunofluorescence imaging for proteins like p53, and assays to measure ROS levels and mitochondrial integrity all help paint the picture of how these drugs affect endothelial health.
Below is a bulleted summary of some experimental observations:
- SA-β-gal Staining: A clear indicator of senescence, with more cells exhibiting blue cytoplasmic staining after exposure to chemotherapeutic agents.
- DNA Damage Markers: Increased levels of 53BP1 and γH2AX indicate that extensive double-strand breaks are being induced.
- Cytoskeletal Disarray: Phalloidin staining often reveals a fragmented and misaligned architecture in drug-treated cells, reflecting compromised cell structure.
- Reduced Proliferation: Lower levels of Ki67, a marker of cell proliferation, are consistently noted, further confirming the senescent state.
- Mitochondrial Markers: Disrupted mitochondrial distribution and morphology are regularly observed, consistent with oxidative stress and ROS accumulation.
These experimental insights are not just academic; they have a clear bearing on clinical practice. When doctors treat cancer patients with potent chemotherapeutic regimens, they must weigh the benefits against potential long-term vascular complications. It becomes a balancing act of ensuring that while cancer is being effectively targeted, the collateral damage to healthy cells does not compromise patient quality of life in the long run.
Working Through the Nitty-Gritty of Mitigating Endothelial Damage
When looking at the various approaches to prevent or reduce chemotherapy-induced endothelial senescence, a few strategies come to light that are both super important and promising:
- Antioxidant Therapy: Introducing mitochondrial-targeted antioxidants like Mito-Q can help clear excess ROS, especially in scenarios where drug toxicity is primarily driven by oxidative stress. This can be critical in preserving endothelial function.
- Drug Combination Strategies: Combining chemotherapy with adjunct treatments that modulate DNA repair mechanisms or antioxidant defenses might offer an effective way to manage the side effects. For example, carefully timed administration of antioxidants could mitigate the damaging bursts of ROS following drug treatment.
- Selective Inhibition: While ATM inhibitors have not yet shown consistent results in reversing drug-induced senescence, further research might optimize how and when these inhibitors are used, especially in combination with other protective agents.
- Personalized Treatment Plans: Recognizing that not all chemotherapeutic agents work in the same way, tailoring cancer treatment to the unique needs of the patient and the specific drug regimen remains key. This individualized approach may help reduce the overall stress on endothelial cells.
Together, these strategies underscore that the path to improved cancer care is full of twists and turns but ultimately findable with rigorous preclinical and clinical research. Clinicians and researchers alike need to make their way through the myriad of options, weighing the benefits of robust anticancer activity against the risk of long-term endothelial damage.
Digging Into the Role of TNF and Inflammatory Signaling Pathways
The senescence process in endothelial cells is not solely about cell cycle arrest and oxidative stress; it is also highly intertwined with inflammation. When cells undergo senescence, they tend to secrete a variety of inflammatory cytokines, including IL-6, IL-8, and TNF-α. These molecules play a key role in shaping the microenvironment of tissues, influencing not only local inflammation but also promoting systemic consequences.
Recent transcriptomic analyses indicate that in methotrexate-treated cells, genes linked to the TNF signaling pathway are significantly upregulated. This observation suggests that beyond its direct DNA-damaging effects, methotrexate may also drive senescence through inflammatory cytokine cascades that further exacerbate mitochondrial dysfunction and ROS overproduction.
This insight into the inflammatory pathways adds another layer of complexity. However, it also opens up additional avenues for intervention, such as targeting specific inflammatory mediators with pharmacological agents or utilizing anti-TNF therapies. The goal here is to reduce the overall inflammatory load, thus mitigating the ripple effects that drive cellular aging.
By incorporating these approaches, treatment regimens can be better refined to not only attack the tumor but also limit the side effects that arise from a full-blown inflammatory response within the vascular system.
Evaluating the Safety and Efficacy of Mitochondria-Targeted Antioxidants
One of the most exciting advances in mitigating chemotherapy-induced damage is the use of mitochondria-targeted antioxidants. Mito-Q, a ubiquinone derivative tailored to accumulate in the mitochondrial matrix, has shown potential in reducing ROS-dependent senescence, particularly in the context of methotrexate treatment. Its mechanism is relatively straightforward: by scavenging excess ROS, Mito-Q can lessen mitochondrial damage and, in turn, blunt the activation of senescence markers such as p21 and inflammatory cytokines (IL-6 and IL-8).
Nevertheless, while the benefits of Mito-Q are promising, its effectiveness is drug-specific. Studies have revealed that its protective role is most pronounced with methotrexate but not with agents like doxorubicin or etoposide, which generate substantial DNA damage independent of ROS. This distinction underscores the importance of recognizing that each chemotherapy drug has its own set of challenging pieces. Therefore, finding your way through the complicated pieces of drug-induced senescence means developing combination strategies that target both ROS and direct DNA damage simultaneously.
Future research should continue to compare the effectiveness of mitochondrial antioxidants with conventional compounds like N-acetylcysteine, aiming to pin down the optimal approach for each clinical scenario. Moreover, the off-target effects and the pharmacokinetics of these compounds need further investigation to ensure that while we protect healthy tissues, we do not dampen the anticancer efficacy of chemotherapy.
Sorting Out the Implications for Long-Term Cancer Survivorship
With cancer survival rates steadily improving, thanks to advancements in chemotherapeutic strategies, the importance of addressing long-term side effects is more critical than ever. Endothelial cell senescence and the resultant vascular dysfunction can predispose survivors to cardiovascular complications later in life, a concern that is both intimidating and filled with potential long-term challenges.
It is essential that oncologists and researchers work together to monitor and manage the subtle details of vascular health in patients undergoing chemotherapy. The battle against cancer does not end with tumor shrinkage; the lingering effects on healthy tissues can have lasting repercussions on quality of life. Clinicians should consider integrating regular cardiovascular monitoring and implementing early intervention strategies for patients identified as being at higher risk of vascular damage.
Some key recommendations for managing long-term toxicity include:
- Regular Screening: Routine imaging and biomarker screening to detect early signs of endothelial dysfunction and oxidative stress.
- Tailored Follow-up: Personalized care plans that include cardioprotective measures tailored to the individual’s treatment regimen.
- Adjunctive Therapies: The strategic use of ROS scavengers and anti-inflammatory agents during and after chemotherapy to preserve endothelial health.
- Lifestyle Interventions: Encouraging survivors to adopt habits that support vascular health, such as regular exercise, balanced nutrition, and stress management.
By taking these steps, the medical community can better steer through the overwhelming repercussions of chemotherapy-induced vascular issues and help ensure that enhanced survival does not come at the price of diminished long-term health.
Taking a Closer Look at the Future of Personalized Cancer Therapy
One of the most promising directions in cancer care today is the shift toward personalized treatment plans. Not all chemotherapeutic agents function in the same way, and not all patients respond identically to these drugs. Understanding the small distinctions in how various agents induce endothelial senescence is key to designing safer medications and treatment plans.
Future clinical protocols may incorporate detailed assessments of a patient’s vascular profile prior to chemotherapy, using biomarkers such as ROS levels, γH2AX foci counts, and expression levels of senescence-related genes like p21, IL-6, and IL-8. Furthermore, genetic screening could help predict which patients are most at risk for developing vascular complications, allowing for a more tailored approach to treatment.
This level of personalization also implies a more nuanced combination therapy, whereby antioxidants, DNA repair enhancers, and anti-inflammatory agents might be administered alongside traditional chemotherapy to temper the overall side effect profile. Multidisciplinary teams consisting of oncologists, cardiologists, and molecular biologists will be essential to make progress in this field, ensuring that every patient receives a treatment plan that is finely tuned to their specific needs.
Charting a Safer Course: The Importance of Continued Research
The research into chemotherapy-induced endothelial cell senescence is still very much a work in progress. Studies employing in vitro models, such as HUVECs, are shedding light on the delicate balance between effective cancer cell killing and collateral damage to healthy tissue. However, translating these findings into clinical practice will require carefully designed trials and longitudinal studies.
Future avenues of research should address several key questions:
- Can the protective effects of mitochondrial antioxidants like Mito-Q be enhanced when combined with other supportive therapies?
- What are the long-term effects of antioxidant adjunct therapy on overall cancer treatment outcomes and patient quality of life?
- How can personalized biomarkers be integrated into clinical decision-making to predict and prevent endothelial damage?
- What role does the inflammatory milieu, especially the TNF signaling axis, play in mediating the persistent effects of chemotherapy, and how can this be modulated effectively?
Answering these questions will require collaboration across various disciplines, as well as a willingness to take a closer look at the nuance in the underlying mechanisms. As more data emerges, the ultimate goal is to develop tailored strategies that preserve the anticancer efficacy of chemotherapy while simultaneously protecting patients from its less desirable effects.
Key Takeaways and the Road Ahead
In summary, chemotherapeutic agents play a double-edged role in cancer treatment. While they are essential in eliminating tumor cells, their impact on endothelial cells—through triggers like DNA damage, ROS accumulation, mitochondrial dysfunction, and inflammatory cascades—presents a set of challenging issues. The following points encapsulate the key messages:
- Understanding HUVEC Senescence: Studies show that chemotherapy can trigger senescence in endothelial cells, impacting vascular health.
- Mechanisms at Play: The damage involves several intertwined factors including ROS overproduction, mitochondrial fragmentation, and activation of DNA damage response proteins like ATM.
- Drug-Specific Effects: Not all chemotherapeutic drugs affect cells in the same way. Methotrexate, for example, relies heavily on ROS production, making it more amenable to interventions with ROS scavengers like Mito-Q.
- Adjunctive Therapy Strategies: A combined approach with antioxidants and anti-inflammatory measures may hold the key to reducing the side effects of chemotherapy.
- Long-Term Implications: With cancer survival rates on the rise, ensuring long-term vascular health is a super important aspect of patient care.
Ultimately, the research community must continue to sort out these issues through careful preclinical studies and well-structured clinical trials. By doing so, we can make more informed decisions on how to minimize the unintended consequences of chemotherapy and enhance the quality of life for cancer survivors.
Final Thoughts on Balancing Efficacy and Safety
While the road to safer cancer treatment is full of twists and turns, the ongoing research into endothelial cell senescence offers hope. It is a reminder that the human body is a finely tuned machine where interventions in one area inevitably affect another. The challenge is to work through the confusing bits and identify strategies that mitigate side effects without undermining the critical task of killing cancer cells.
As we continue to piece together the puzzle of chemotherapy-induced senescence, it is essential to appreciate the delicate balance between drug efficacy and the preservation of healthy tissues. This balance is not only critical for immediate treatment outcomes but also for ensuring that survivors do not face overwhelming vascular problems in later years. With the collective efforts of the scientific and medical communities, we can aspire to a future where cancer treatments are as kind to healthy cells as they are fierce against tumors.
Concluding Remarks
In conclusion, the emergence of oxidative stress and endothelial senescence as byproducts of chemotherapy highlights the need for a more nuanced approach to cancer treatment. Although the challenges are many and loaded with issues, targeted strategies such as the application of mitochondrial antioxidants and personalized treatment protocols show great promise. As the field continues to evolve, a careful balance between attacking cancer and protecting the body’s vital networks must be maintained.
For now, clinicians and researchers alike must continue to take a closer look at the fine points of these processes, using the insights gained to guide the development of next-generation therapies. By working through these complicated pieces with precision and care, the future of cancer treatment can be both effective and considerate of long-term patient health.
This opinion editorial is not just a reflection on current findings but also a call to action—a reminder that continuous research and innovative thinking are critical to ensuring that our fight against cancer does not inadvertently compromise the systems that keep us alive and thriving.
Originally Post From https://www.nature.com/articles/s41598-025-20652-z
Read more about this topic at
Diversity of oxidative stress and senescence phenotypes …
Chemotherapy-induced senescence, an adaptive …
