Understanding Notch4’s Essential Role in Pituitary Vascular Homeostasis
The study of Notch4 signaling in the maintenance of vascular networks, particularly in the young adult pituitary posterior lobe, has taken center stage in recent biomedical discussions. This opinion piece takes a closer look at how Notch4 deficiency and its inhibition with agents like DAPT affect vascular density, branch length, and overall vessel integrity, and what these findings might mean for both current therapeutic strategies and our understanding of endocrine regulation.
At the heart of this discussion is the finding that the adult pituitary vasculature appears to retain characteristics of immature vessels. Unlike most adult blood vessels that are generally stable and less responsive to growth factor fluctuations, these vessels show a surprising sensitivity to Notch inhibition. This suggests that, even in adulthood, there are unique layers of regulation that remain seemingly tied to the developmental phase, offering new perspectives on tissue-specific vascular regulation.
Notch4 Signaling and Its Impact on Vascular Integrity
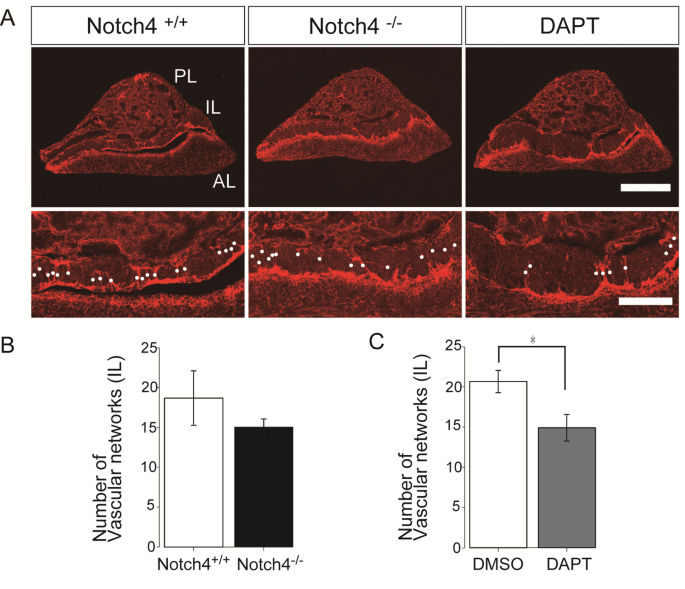
Notch4 has long been recognized as a major player in vascular morphogenesis, but its role in the maintenance of stable vessels in a mature organ such as the pituitary is particularly fascinating. Researchers have recently observed that reducing Notch4 activity causes a decrease in vascular density, branch length, and the number of branch points—effectively changing the landscape of blood vessel architecture. This has set off a debate about whether these changes reflect only a developmental shortcoming or if they point to an ongoing requirement for Notch signaling in keeping vessels balanced.
The observed vascular alterations in Notch4-deficient mice underscore a few important themes:
- Reduced vascular density may compromise the efficient exchange of nutrients and hormones.
- The loss of branch complexity could mean that the vascular network loses its flexibility to respond to local changes.
- Changes in vessel radius, particularly the loss of larger-caliber vessels, may lead to shifts in the identity of blood vessels—from arteries to more venous-like patterns.
These tough but crucial observations highlight that the maintenance of Notch4 activity is more than a backup for embryonic development—it is also a key, ongoing factor in adult vascular health.
Comparing Notch4 Deficiency With DAPT-Mediated Notch Inhibition
A notable part of the research has been comparing genetically induced Notch4 deficiency with the pharmacological inhibition of Notch signaling using DAPT, a gamma-secretase inhibitor. The use of DAPT to block Notch signaling reveals similar patterns of vascular disruption: reduced density, decreased branch length, and a distinct loss of larger radius vessels. However, the differences in the exact changes—such as the differing lengths of the affected vessels—point towards a complex interplay between the intracellular and extracellular domains of Notch4.
This comparison gives rise to several intriguing points:
- DAPT treatment mimics many, but not all, of the changes seen in Notch4 knockout models.
- The subtle differences in vessel length among larger radius vessels hint at additional roles played by the extracellular domain of Notch4.
- Both genetic and pharmacological models underscore that adult pituitary vessels continue to rely on Notch signaling, even though mature vessels elsewhere may be less affected by similar interventions.
These findings prompt us to consider that while overall the trends are consistent, the underlying biological mechanisms may involve more tangled issues and subtle differences that require further study.
Assessing the Vascular Health in a Tissue-Specific Context
Perhaps one of the most thought-provoking implications of this research is the suggestion that Notch4’s role in maintaining the endocrine vasculature is very tissue-specific. The adult pituitary posterior lobe, as opposed to other mature tissues, retains many traits of its developing partners, such as heightened sensitivity to VEGF (vascular endothelial growth factor) fluctuations. This raises several questions:
How do these adult vessels maintain a responsiveness similar to immature vessels? What does it mean for endocrine functions if the homeostasis of these vessels is disrupted? And finally, how could future therapies inadvertently affect this delicate system?
The fact that vascular homeostasis can be so dramatically affected by changes in Notch signaling emphasizes the need for precision when designing therapeutic strategies—especially those targeting Notch receptors in chronic diseases or cancer treatment.
Exploring the Dual Role of Notch4 in RBP-J-Mediated Transcription
Central to the discussion is the dual nature of Notch4. The intracellular domain of Notch4 is responsible for transactivating RBP-J-mediated transcription, while its extracellular domain appears to inhibit ligand-induced activation of Notch1. The opposing actions of these two domains play into the “tangled issues” that have been mentioned in the scientific community.
When Notch4 is missing, the loss of the intracellular domain’s function appears to lead to a downstream upregulation of NR2F2—a key marker associated with venous vessels. This upregulation signals a deficiency in the arteriovenous identity of the pituitary vasculature, which, in turn, could compromise the functionality and integrity of the vascular network. In simple terms, without the balancing act provided by Notch4, the normal cues that help blood vessels maintain an arterial or venous identity become skewed.
This discovery is critical because it frames Notch4 not just as a developmental regulator but as an ongoing essential factor in adult circulatory integrity. Despite significant research in other tissues, these findings remain somewhat unique to the pituitary and may open up new avenues for understanding vascular maintenance in endocrine organs.
Delving Into the Study’s Methods: Automated Vascular Image Analysis
An equally fascinating aspect of this research lies in its methodology. Researchers employed an automated vascular image analysis workflow using modalities such as skeletonization and Euclidean distance maps. This approach allowed the team to quantify several parameters with clarity and precision:
| Parameter | Description |
|---|---|
| Vascular Density | The proportion of the vascular area to the total tissue area. |
| Branch Length | Total length of vessels measured from the skeletonized image. |
| Number of Branching Points | The number of intersections or splits in the vascular network. |
| Branch Radii | The distance from the vascular centerline to the vessel wall, measured for different vessel sizes. |
This level of quantitative analysis is critical in distinguishing the subtle differences between normal and altered vascular architecture. It also paves the way for more robust comparisons between genetic models (like Notch4 knockout) and pharmacologically induced models (using DAPT). Such tools are a must-have for modern biomedical research because further improvements in imaging technology could help steer through the tangled issues currently faced in vascular biology.
Investigating The Tricky Parts of Arteriovenous Identity
One of the more intimidating challenges in vascular biology is understanding how blood vessels decide their fate—to become an artery or a vein. In the case of the adult pituitary, the loss of Notch4 or the application of DAPT both led to a reduction in larger radius vessels. Upon closer examination, these vessels were found to be positive for Dll4, a marker traditionally associated with arterial identity, yet they were also accompanied by an increase in NR2F2 expression, a marker for venous identity.
This observation presents a confusing bit of the puzzle. It suggests that when Notch4 signaling is disrupted, vessels lose a clear-cut identity, becoming a mix of arterial and venous traits. The potential consequences of such arteriovenous identity deficiency may be far-reaching, touching not only on vascular function but also on the overall endocrine regulation within the pituitary.
In short, the loss of clear vessel identity is a serious challenge that invites researchers to dig into the fine points of these signaling pathways further. The nuances involved here are more than just academic details; they have direct implications for how we might approach vascular repair and regeneration in the future.
Implications for Endocrine Function and Disease Treatment
The pituitary gland is central to hormonal control, and its vascular integrity is essential for proper endocrine function. Given that the pituitary vasculature is responsible for efficient hormone release and nutrient exchange, any disruption to this delicate network can have wide-ranging impacts on the body’s overall health.
When the vascular network is compromised, several potential issues may arise:
- Hormonal Imbalance: Reduced nutrient and oxygen supply can lead to suboptimal hormone secretion, impacting metabolism, stress responses, and reproductive functions.
- Structural Changes: A reduction in branch points and vessel caliber may affect the structural support needed for the pituitary, potentially leading to tissue dysfunction.
- Secondary Complications: Vascular disruption might pave the way for further pathological changes, including hypoxia-related damage or even tumorigenesis under certain conditions.
The recognition that Notch4 signaling plays a critical and nuanced role in maintaining the endocrine-associated vasculature is not just of academic interest—it also raises red flags about some of the therapeutic approaches being explored. For example, anti-Notch4 therapies that show promise in tumor treatments could inadvertently compromise the vascular integrity of endocrine organs like the pituitary, leading to unwanted side effects.
Tissue-Specific Vascular Regulation: More Than One Size Fits All
A recurring theme in the debate on Notch4 is the idea that not all tissues are created equal. The pituitary posterior lobe, with its immature vascular characteristics, differs significantly from more mature vascular systems found elsewhere in the body. Other organs, such as the retina, show different responses to Notch inhibition, where arterial barrier functions may be affected without disturbing arteriovenous identities.
This tissue-specific response is a reminder that while the broad strokes of Notch signaling might be conserved, the little twists and subtle differences in each tissue’s response to Notch inhibition can be considerable. The complexity of these differences makes it clear that therapeutic strategies must be designed with an intimate understanding of tissue-specific vascular regulation.
A visual summary of these tissue-specific factors might look like this:
| Feature | Pituitary Posterior Lobe | Retinal Vasculature |
|---|---|---|
| Sensitivity to Notch Inhibition | High, with marked effects on vascular density and branch complexity. | Moderate, with arterial barrier functions more affected than overall identity. |
| Response to VEGF | Retains features of immature vessels, showing high VEGF responsiveness. | Typically more mature, with VEGF signals supporting normal homeostasis. |
| Tissue Function | Directly linked to hormonal regulation and endocrine balance. | Critical for maintaining the blood-retinal barrier integrity. |
Such tables help digest the tricky parts of tissue-specific differences, emphasizing why a generic approach to Notch signaling might fall short of addressing the local problems uniquely seen in endocrine tissues.
Looking Into Therapeutic and Clinical Applications
The idea that interfering with Notch signaling could affect vascular structure raises important questions for clinicians. With the rising interest in utilizing anti-Notch therapies in the treatment of various cancers, especially those that depend on robust angiogenesis for their growth, caution is needed. Notch4 inhibition, for instance, may offer the advantage of stifling tumor growth by limiting blood flow; however, its systemic effects on endocrine functions cannot be ignored.
Clinicians and researchers alike must now consider a few critical facets when designing interventions:
- Risk of Endocrine Disruption: Inhibiting Notch4 could inadvertently harm the pituitary’s complex vascular network, leading to compromised hormonal balance.
- Tissue-Specific Sensitivity: Since the pituitary is more like an immature vascular bed, therapies need to be refined to avoid off-target effects in endocrine tissues.
- Therapeutic Windows: Finding the right dose and timing for Notch inhibitors is key—too much inhibition and the effects could be overwhelming, too little may not yield the desired anticancer benefits.
It is therefore paramount that future clinical trials include careful assessments of vascular integrity in endocrine organs in addition to the primary target tissues. In doing so, clinicians can ensure that the twists and turns encountered during therapy do not lead to unexpected consequences.
Decoding the Nitty-Gritty of Endothelial-Specific Notch Signaling
One of the current challenges is the possibility that the effects observed with Notch4 deficiency or DAPT inhibition might occur not solely through the traditional canonical Notch pathways, but potentially via alternative, non-canonical routes as well. In simpler terms, while the loss of RBP-J-mediated transcription gives us one clue, there may be other routes by which the absence of Notch4 leads to vascular disruption.
This raises a number of questions and research opportunities:
- Is the upregulation of NR2F2 simply a side effect of Notch signaling inhibition, or is it a primary driver of the observed vascular changes?
- Could there be alternative pathways within the endothelial cells that are activated once Notch4 is removed?
- Would a targeted, endothelial-specific knockout of Notch4 provide further insights into these subtle parts?
To answer these tricky questions, scientists are considering the use of endothelial-specific RBP-J conditional knockout models. Such experiments would allow researchers to poke around the fine details of how Notch signaling functions in a cell-specific manner, separating the direct effects from secondary or systemic responses.
Future Directions and the Road Ahead
The current debate on Notch4 in the pituitary vasculature leaves many avenues for further research; the path is full of twists and turns that are as exciting as they are complicated. Future studies must address these key questions:
- Endothelial-Specific Mechanisms: Utilizing models that target only endothelial cells can help isolate the cell-autonomous effects of Notch4 deficiency.
- Therapeutic Refinement: If Notch4-targeted therapies move forward in clinical settings, their impact on endocrine vasculature will need careful evaluation.
- Cross-Tissue Comparisons: Studying the differences between the pituitary, retina, and other vascular beds may reveal insights into why some tissues are more vulnerable than others.
- Anticipating Off-Target Effects: As anti-angiogenic therapies become more common in cancer treatment, understanding the systemic nature of Notch signaling inhibition is critical for avoiding nerve-racking side effects.
It is also worth noting that in the broader context of modern medicine, alternative approaches such as targeted gene therapy or selective modulation of signaling pathways may offer a way to manage these complicated pieces without compromising overall vascular health. A detailed understanding of the fine points of Notch signaling, and especially how Notch4 contributes to vascular stability, will be key to designing such strategies.
Rethinking Vascular Maintenance in the Context of Modern Endocrinology
The endocrine system is a finely tuned network where vascular health plays a super important role. When the blood vessels, especially within the pituitary, start to show signs of stress or remodeling due to inhibited Notch4 signaling, the body’s overall balance may be thrown off. Hormone levels, nutrient delivery, and waste removal can all be affected, leading to systemic issues that no specialist wants to encounter.
Considering this connection, it becomes evident that interventions targeting Notch signaling in any context—oncological or otherwise—must be approached with an eye on holistic vascular health. The endothelial cells of the pituitary, with their immature attributes and dependence on VEGF, remind us that even in adult tissues, there exist hidden complexities that demand careful management.
In a clinical context, this means that while anti-angiogenic treatments might help reduce tumor growth, they could inadvertently trigger endocrine dysfunction. The notion should give pause to clinicians and researchers, urging them to sort out these issues early in the research and development process.
Integrating Modern Imaging Techniques to Illuminate Vascular Dynamics
One of the cutting-edge advancements in this field is the integration of automated vascular image analysis. By leveraging techniques such as image skeletonization and Euclidean distance mapping, researchers can now quantify vascular parameters in an unprecedentedly detailed manner. This not only allows scientists to measure the overt changes in vascular density and branch architecture, but also to appreciate the subtle parts—those minute changes that might otherwise go unnoticed.
A summary of the imaging workflow includes:
- Image Acquisition: High-resolution confocal microscopy captures the intricate network of blood vessels stained with endothelial markers.
- Image Processing: Software such as Fiji is used to apply filters and extract the vascular network, turning raw images into quantifiable data.
- Quantification: Parameters such as branch density, branch length, and branch radii are systematically measured, providing insight into how Notch4 deficiency impacts vascular structure.
This combination of technology and biology is not just fascinating—it is a necessary tool that modern researchers must use to figure a path through the complicated pieces of vascular regulation.
Addressing The Overwhelming Complexity of Vascular Biology
For both researchers and clinicians, the task of understanding and ultimately controlling vascular functions in the pituitary and other endocrine organs is filled with intimidating challenges. The sensitive interplay of growth factors like VEGF, the balance maintained by Notch signaling, and the requirement for clear arteriovenous identity all add layers of complexity that can seem overwhelming at first glance.
However, by carefully examining each component in isolation and then synthesizing the data, scientists can piece together a clearer picture of how these systems work. It is important to remember that while the experiments described may portray a somewhat nerve-racking environment of competing signals and roles, each step taken in the research process brings us closer to more refined therapies and a better understanding of human physiology.
Even the areas that seem laden with tricky parts and tangled issues are opportunities for discovery. As research continues, the innovative integration of genetic models with sophisticated imaging techniques promises to provide the clarity needed to manage your way through these challenges.
Learning From Cross-Disciplinary Insights
Another key consideration is the value of cross-disciplinary research in this domain. Vascular biology does not operate in isolation—its insights can often be enriched by findings from related fields such as endocrinology, oncology, and even computational image analysis. For example:
- Endocrinologists are learning that even subtle changes in vascular architecture can have profound impacts on hormone distribution and overall endocrine balance.
- Oncologists are increasingly cautious about the side effects of anti-Notch4 therapies, recognizing that targeting one pathway might ripple across multiple tissue systems.
- Imaging specialists continue to refine automated methods to trace the fine shades of vascular disruption, providing indispensable data to guide clinical decisions.
By pooling together these perspectives, the medical community can better appreciate the full spectrum of effects when shifting the balance of Notch signaling. The interdisciplinary nature of the challenge means that no single field holds the answer, but together, they offer a multifaceted view that promises more effective solutions in the long run.
Concluding Thoughts: Charting a Course for Future Research
In summing up, the research on Notch4’s role in maintaining the vascular homeostasis of the pituitary posterior lobe is a vivid reminder of the ongoing challenges in modern vascular biology. The data suggest that the adult pituitary vasculature, with its immature and VEGF-sensitive characteristics, relies heavily on Notch4 signaling for structural and functional maintenance. Both genetic deficiency and pharmacological inhibition using DAPT reveal similar patterns of disruption, albeit with some nuanced differences that point to the dual roles of Notch4’s intracellular and extracellular domains.
This wealth of information, full of tricky parts and tangled issues, underscores not only the need for continued inquiry but also a cautious approach to therapeutic interventions targeting Notch signaling. Researchers must now work collaboratively to figure a path through these complexities, ensuring that the potential benefits of Notch-targeted therapies do not come at the cost of endocrine dysregulation.
Looking ahead, several action items are clear:
- Develop endothelial-specific models to isolate the direct effects of Notch4 deficiency.
- Refine imaging and quantification methods to capture the small distinctions in vascular architecture.
- Examine the broader systemic implications of disrupting Notch signaling beyond the target tissue.
- Integrate cross-disciplinary insights to guide therapeutic strategies and mitigate unintended side effects.
By addressing these action items, the scientific community can pave the way for interventions that respect the delicate balance of endocrine vascular systems while still offering new hope in the fight against cancer and other diseases where vascular growth is a key factor.
Ultimately, the debate over Notch4 in the pituitary is not simply an academic exercise—it is a critical conversation that carries substantial clinical significance. As we continue to dig into the hidden complexities of vascular regulation, each incremental discovery is a stepping stone towards safer, more effective treatments that honor the intricate interplay of cellular signaling, tissue specificity, and overall organismal health.
Final Reflections on the Future of Notch4-Targeted Therapies
The insights gained from these studies shine a light on a future where personalized medicine and precision therapies could embrace the full spectrum of vascular regulation. Therapeutic strategies that might once have seemed overly intimidating can now be refined, thanks in large part to the advanced imaging techniques and genetic models at our disposal. The approach of combining automated quantitative analysis with traditional histological methods is set to revolutionize the way we understand and treat vascular disorders.
As researchers and clinicians make their way through the complicated pieces of cellular signaling, the focus must remain on balancing effective treatment with the preservation of essential endocrine functions. In this journey, keeping a close watch on components such as Notch4 will be essential, not only to appreciate its critical role in the pituitary but also to avoid the off-target effects that could compromise the well-being of patients.
The conversation around Notch signaling, especially in a tissue as delicate as the pituitary, continues to evolve. With ongoing research efforts aimed at understanding the fine points of Notch4’s dual roles, there is a palpable sense of anticipation about what the next wave of studies will reveal. Every new finding brings us a little closer to a future where therapies are not just effective but also carefully calibrated to the nuanced requirements of each tissue.
While the twists and turns of this research field may seem overwhelming at times, they also offer hope. The ability to dig into the small distinctions of vascular behavior assures us that, with persistence and collaboration across disciplines, the challenges currently faced can be successfully navigated. It is a reminder that in the realm of modern medicine, even the most intimidating puzzles eventually yield insights that can transform patient care.
In conclusion, the role of Notch4 in the adult pituitary vascular homeostasis is a vivid example of how nuanced and tissue-specific biological processes can be. From the changes in vessel density and branch complexity to the emerging understanding of arteriovenous identity, this research weaves together multiple strands of inquiry. As we continue to take a closer look at the interplay between Notch signaling and vascular regulation, it is clear that the shot at designing effective, safe therapies depends on our ability to appreciate and respect the delicate balance inherent in our bodily systems.
Only by recognizing the essential nature of these signaling pathways—and by continuing to refine our methods of analysis—can we hope to deliver interventions that maintain the health of our vascular networks while addressing the needs of patients suffering from a host of challenging conditions. The journey ahead is filled with both opportunities and challenges, but it is one that holds the promise of transforming how we treat vascular and endocrine disorders for generations to come.
Originally Post From https://www.nature.com/articles/s41598-025-17225-5
Read more about this topic at
Notch4 is essential for the maintenance of vascular …
Molecular Determinants of NOTCH4 Transcription in …


