The Essential Role of VEGF Signaling in Health and Disease
Vascular Endothelial Growth Factor (VEGF) signaling is a key pathway in our bodies that regulates important processes like blood vessel formation, wound healing, and even the maintenance of the tissue environment. As we get deeper into the topic, the role of VEGF may look intimidating due to its many self‐regulatory loops and the array of receptors it engages with; however, by taking a closer look, one can figure a path through these confusing bits and understand its crucial, or in fact, must-have contributions to both health and disease.
The VEGF family consists of several proteins that come in different flavors and sizes. These include VEGF-A, VEGF-B, VEGF-C, VEGF-D, and Placental Growth Factor (PlGF). They engage with different receptors (VEGFR1, VEGFR2, and VEGFR3) and co-receptors, offering a tangled network of signals that can stimulate new blood vessel growth (angiogenesis) and lymphatic development (lymphangiogenesis). Even though the molecular bonding and the downstream mediators seem loaded with problems, examining the fine points can help us understand how these molecules work both in normal physiology and in various diseases.
Decoding the Tricky Parts of Molecular Interactions
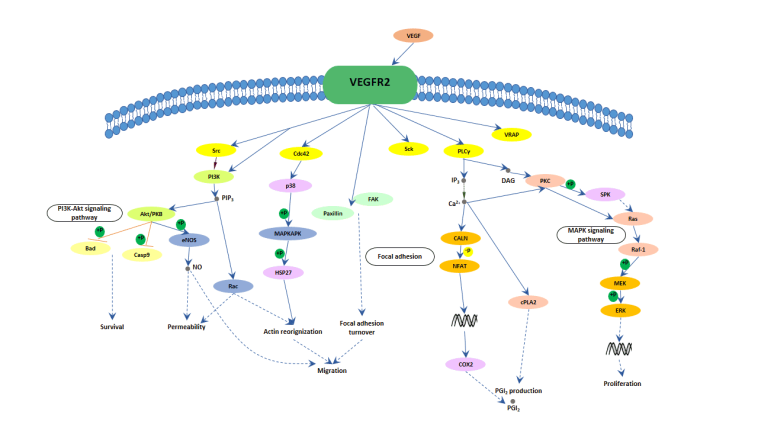
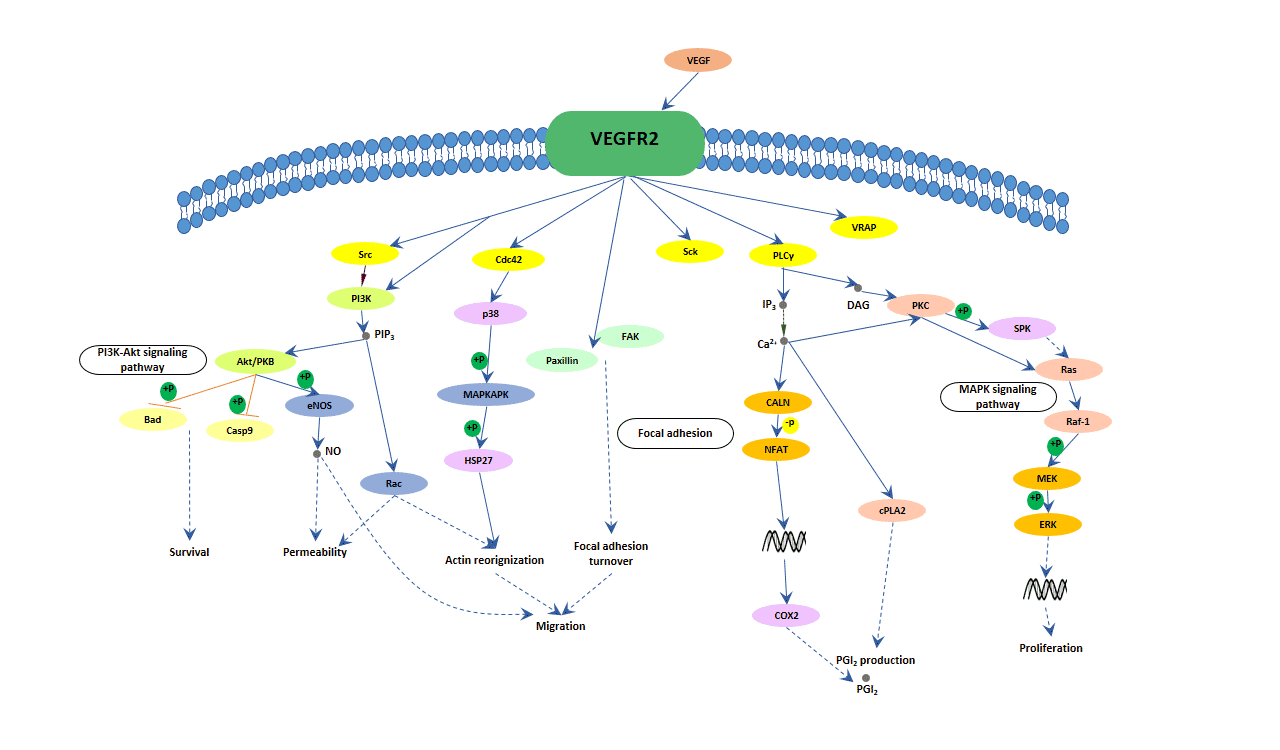
One of the most interesting yet complicated pieces of the VEGF puzzle is how these growth factors bind to their receptors. When VEGF molecules attach to receptors on the cell surface, they trigger a cascade of intracellular signals. This process, while critical, involves twists and turns that are sometimes nerve-racking to work through.
Researchers have found that each VEGF isoform has slightly different features. For instance, VEGF-A is known for its potent angiogenic effect, and small distinctions in its structure can affect how strongly it interacts with VEGFR2. By contrast, VEGF-B, although less celebrated for its angiogenic power, plays a super important role in balancing the survival and metabolism of blood vessels. These subtle details are what scientists are now trying to poke around – diving into not just the main signaling but also the hidden layers of receptor regulation, such as the role of receptor dimerization and the impact of post-translational modifications.
Molecular Bonding and Downstream Signaling
When VEGF binds to a receptor like VEGFR2, it sets off a chain reaction that includes the activation of intracellular enzymes and the phosphorylation of specific tyrosine residues. These steps are critical for stimulating cell division, migration, and the remodeling of the blood vessels. Here is a simplified bullet list summarizing some key steps:
- VEGF binding to its receptor initiates receptor dimerization.
- Dimerization triggers autophosphorylation, activating several kinase pathways.
- This activation ultimately leads to gene expression changes and cell proliferation.
- The downstream signaling also affects processes like vascular permeability and repair.
Although these pathways seem to be straightforward at first glance, they are interwoven with negative feedback loops and degrading factors that help the cell find its balance – factors that might be described as the nitty-gritty of the signaling network.
The Tumor Microenvironment and the Tangled Issues of Angiogenesis
In the context of cancer, VEGF signaling acquires an even more complicated layer. Tumors often secrete high levels of VEGF to fuel their own blood supply and support rapid growth, which in turn creates a microenvironment full of trick questions and perplexing feedback loops. For many, understanding the dynamics between angiogenesis and immune cell infiltration in tumors is a nerve-racking challenge.
Researchers have discovered that excess VEGF not only stimulates new blood vessel proliferation but also makes existing vessels leaky, which may enhance metastatic spread. In tumors, these fine shades of VEGF signaling can be both an advantage—as they allow for nutrient and oxygen supply—and a drawback, as they create inflammatory signals that further complicate treatment.
Cancer-Related VEGF Challenges
Some specific tangled issues within tumor angiogenesis include:
- Aberrant Vessel Formation: Tumor blood vessels formed under high VEGF conditions are often irregular and disorganized. These vessels are more permeable, which can lead to increased interstitial fluid and a subsequent rise in the pressure within the tumor.
- Therapeutic Resistance: Anti-angiogenic therapies (for example, bevacizumab) target VEGF pathways to starve the tumor. However, tumors may develop resistance by upregulating alternative angiogenic factors, making treatment approaches seem off-putting to manage.
- Immune Evasion: The VEGF-driven tumor environment is also known to interfere with immune cell recruitment, which adds another layer of small distinctions and turns that complicate efforts to stimulate anti-tumor immune responses.
The presence of such messy or tangled processes in the tumor microenvironment underscores the importance of combining treatment regimens that target multiple pathways to effectively curb tumor growth.
Cardiovascular Implications: Finding Your Path Through VEGF’s Role in Blood Vessel Health
Beyond tumors, VEGF signaling is absolutely essential in the cardiovascular system. Blood vessel maintenance, vascular repair, and even blood pressure regulation are intricately linked to the VEGF/VEGFR axis. Although the underlying mechanisms might look intimidating due to the complexity of vascular biology, many of these processes are critical for survival.
For example, within the heart and blood vessels, VEGF-A and VEGF-B play key roles in preserving the integrity and function of capillaries. The process by which VEGF influences endothelial cells (cells lining the blood vessels) involves several steps:
- Endothelial Cell Survival: VEGF signaling helps these cells avoid apoptosis (cell death) and supports the repair of damaged vessels.
- Vascular Permeability: While a controlled level of permeability is essential for nutrient exchange, too much can lead to swelling and inflammation, adding another tricky part that interacts with other regulatory systems.
- Blood Pressure Modulation: VEGF pathways intersect with signals that control the production of nitric oxide, a super important molecule that dilates blood vessels and keeps blood pressure in check.
Below is a simple table that outlines how VEGF signaling can affect various aspects of cardiovascular health:
| Aspect of Vascular Health | VEGF-Related Effect |
|---|---|
| Endothelial Cell Survival | Promotion of anti-apoptotic signals and repair mechanisms |
| Vascular Permeability | Controlled leakiness for nutrient exchange, but overactivation can cause edema |
| Blood Pressure Regulation | Stimulation of nitric oxide production, leading to vasodilation |
| Structural Maintenance | Support of vessel stability through interactions with the extracellular matrix |
This table helps illustrate the key areas where VEGF signaling is not only critical, but also where its small distinctions and fine shades might influence therapeutic decisions, especially in conditions such as hypertension, myocardial ischemia, or heart failure.
VEGF Signaling in Eye Disorders: Diving Into the Confusing Bits of Retinal Health
The eye is another organ where VEGF takes on a super important role. In conditions like diabetic retinopathy and age-related macular degeneration (AMD), VEGF signaling often becomes deregulated, leading to the formation of abnormal, leaky blood vessels.
When the retina experiences low oxygen conditions, VEGF levels climb, setting off an overproduction of new vessels. However, rather than being beneficial, this overabundance of vessels can damage the delicate retinal structure. The result is vision loss, a situation that demands therapies capable of calming these nerve-racking, high VEGF environments.
Retinal Diseases and Abnormal Vascular Leakage
In diabetic retinopathy, for instance, the following points highlight the extreme effects of overactive VEGF signaling:
- Excess Vascular Permeability: When VEGF is overexpressed, it can increase the permeability of retinal vessels, causing fluid leakage and macular edema.
- Uncontrolled Neovascularization: The new vessels that form are typically fragile and prone to bleeding.
- Compromised Barrier Integrity: The delicate blood-retinal barrier becomes compromised, intensifying the damaging effects.
Modern treatments, such as anti-VEGF injections, target these wrong or off-target signals. However, managing these treatments can be overwhelming at times. The patient and the clinician need to figure a path through repeated injections and monitor subtle distinctions in improvements versus side effects.
Therapeutic Perspectives: Combining Treatments to Manage Your Way Through a Nerve-Racking Landscape
One of the most promising angles in current biomedical research is the development and integration of therapies aimed at modulating VEGF signaling. Anti-angiogenic drugs have become a cornerstone in the treatment of cancers and ocular diseases. However, as the evidence shows, the interplay between VEGF and other signaling molecules is full of problems and alternative routes. This demands a combination of therapies rather than relying on a single strategy.
When it comes to clinical applications, here are a few important points to consider:
- Combination Therapy: Pairing anti-VEGF agents with other treatments (such as immune checkpoint inhibitors) can help counteract resistance. This approach seeks to tackle the tangled issues from multiple angles.
- Personalization: Not every patient’s VEGF pathway is the same. Tailoring treatment based on patient-specific markers can help manage these fine points more effectively.
- Addressing Side Effects: While reducing VEGF signaling can help starve tumors or fix leaking retinal vessels, it can also lead to side effects like hypertension or impaired wound healing. Thus, understanding the hidden complexities and subtle parts of VEGF’s role in normal tissue is critical.
Consider the following table that summarizes some current therapeutic strategies:
| Therapeutic Agent | Action | Common Applications |
|---|---|---|
| Bevacizumab | Anti-VEGF-A antibody that reduces abnormal vessel growth | Cancer, AMD, Diabetic Retinopathy |
| Aflibercept | VEGF-Trap; binds VEGF-A and other related growth factors | Colorectal cancer, Eye diseases |
| Ramucirumab | VEGFR2 inhibitor that blocks downstream angiogenic signals | Gastric cancer, Lung cancer |
| Ranibizumab | Fragment targeting VEGF-A for intraocular injection | Wet AMD, Diabetic Macular Edema |
These examples show that while VEGF signaling itself is a central player, fine-tuning the therapeutic approach to each patient is crucial amid the twists and turns of the signaling network.
Addressing the Overwhelming Landscape of VEGF-Related Disorders
For researchers, clinicians, and patients alike, the world of VEGF signaling is a mix of essential insights and challenges. Whether it is the task of figuring out how to best manage diabetic complications, harnessing anti-tumor responses in cancer, or preventing vascular leakage in the eye, this pathway sits at the intersection of many different physiological systems.
At the molecular level, the fine details such as receptor dimerization, the interplay with co-receptors like neuropilin, and post-translational modifications such as phosphorylation, glycosylation, SUMOylation, and ubiquitination add layers of interpretation that can seem both overwhelming and fascinating. Tackling these subtle parts is not just a matter of academic interest—it can lead to improved therapeutics and better outcomes for patients facing a range of conditions.
Here is a brief outline of some of the key areas where VEGF’s role is both essential and challenging:
- Cancer: Overactive VEGF signaling contributes to both chaotic vessel formation and immune evasion, requiring combination therapies to counteract these effects.
- Cardiovascular Disease: VEGF signaling supports vessel repair and maintenance, but fine shades in regulation can have implications in conditions like hypertension.
- Ocular Disorders: In age-related macular degeneration and diabetic retinopathy, the balance of VEGF levels is key to preserving vision while preventing abnormal vessel growth.
- Metabolic Disorders: In diabetes and its complications, VEGF plays a double-edged role; it is necessary for islet function and repair, yet its dysregulation can exacerbate tissue damage.
- Inflammatory Conditions: Diseases like rheumatoid arthritis and psoriasis involve inflammatory triggers that modulate VEGF levels, adding a layer of subtle differences that can impact tissue inflammation and repair.
- Reproductive Disorders: Preeclampsia and endometriosis are conditions where the regulation of VEGF and its soluble receptors is critical, with a delicate interplay that can be full of problems if the balance shifts.
Finding a Path Forward: Combining Research with Clinical Application
To steer through the royalty of VEGF signaling problems, future research must continue to investigate the small distinctions in receptor interactions, feedback regulation, and post-translational modifications. Recent advances in genomics, proteomics, and single-cell analyses are rapidly unveiling the nitty-gritty details that were once hidden in the tangled issues of VEGF’s action.
Here are several promising strategies that the scientific community is currently exploring:
- Multi-Modal Therapy: Combining anti-VEGF drugs with immune checkpoint inhibitors, other tyrosine kinase inhibitors, or even genetic therapies to attack the problem from various angles.
- Personalized Medicine: Using biomarkers to tailor treatments based on individual patient profiles. This strategy involves taking a closer look at each patient’s unique VEGF receptor status and related signaling patterns.
- Post-Translational Modulation: Research on how modifications like SUMOylation, ubiquitination, and glycosylation affect receptor function could offer new drug targets to fine-tune VEGF signaling.
- Alternative Targeting: Exploring the inhibition or modulation of co-receptors (e.g., neuropilin) or decoy receptors can help manage the overwhelming excess of VEGF in certain diseases.
As these approaches progress, surely it will be possible to figure a path through the maze of VEGF-related therapies, ensuring that treatments are not only effective but also safe and well-tolerated by patients.
Challenges and the Future of VEGF Research
Despite the enormous strides made in understanding VEGF signaling over the past few decades, there remain confusing bits and intimidating challenges. The field is laden with issues such as drug resistance in cancer treatment, potential side effects in anti-VEGF therapies (like hypertension and renal injury), and the dual roles that VEGF plays in both normal physiology and disease.
Researchers need to work through these tricky parts using both innovative experimental designs and advanced computational models. For instance, figuring a path through the post-translational modifications and the dynamic balance between angiogenesis and vascular stability in various tissues is super important. When these subtle parts are pieced together, they offer tremendous opportunities for the development of next-generation therapies.
The future of VEGF research is likely to focus on:
- Advanced Biomarker Discovery: With the rise of high-throughput technologies, identifying small distinctions in a patient’s VEGF signaling profile could help predict responses to treatment and allow for early intervention.
- Smart Combination Therapies: Rather than relying on a single agent, future approaches may integrate multiple drugs that target different nodes in the VEGF pathway, thereby bypassing or managing drug resistance.
- Gene Editing and RNA Technologies: CRISPR and RNA-based interventions might soon be applied to modulate the expression or splicing of VEGF and its receptors, offering a way to fine-tune the pathway at its source.
- Personalized Treatment Plans: By understanding a patient’s unique molecular makeup, clinicians can better decide which combination of treatments is best suited to manage the nerve-racking intricacies of VEGF signaling.
Conclusion: Embracing the Hidden Complexities with a Balanced Perspective
In summary, VEGF signaling is an essential, multifaceted pathway that affects many aspects of our health—from the growth and maintenance of blood vessels to its implications in diseases like cancer, diabetic complications, and even inflammatory disorders. Although the molecular interactions include many confusing bits and intimidating complexities, ongoing research is steadily uncovering the fine details and hidden complexities that govern this essential pathway.
By taking the time to dive in and get into the twist and turns of VEGF signaling, researchers and clinicians alike are managing their way through a landscape that is full of both promise and challenges. While the journey may seem laden with tangled issues, the progress made so far underscores that a balanced, multi-pronged approach is the key to harnessing the therapeutic potential of VEGF modulation.
From combining anti-VEGF drugs with immune therapies to tracking subtle post-translational modifications, the future of treatment strategies is bright. These advancements, though they may involve messy and nerve-racking challenges, promise to offer improved outcomes for patients facing some of today’s most serious health conditions.
Ultimately, the science behind VEGF signaling is a fine example of how even the most complicated pieces of biology can be understood with perseverance and innovation. By finding a path through the twists and turns, we can develop treatments that not only target disease but also preserve the essential roles that VEGF plays in health. With ongoing advancements, the journey of decoding VEGF signaling continues—paving the way for a future where the tricky parts of molecular interactions are transformed into keys for therapeutic success.
Key Takeaways
- VEGF signaling is critical in normal physiology and in conditions like cancer, heart disease, and eye disorders.
- The molecular interactions involve receptor dimerization, phosphorylation, and numerous post-translational modifications that provide a tangled yet essential regulatory network.
- Cancer treatments often face resistance as tumors use alternative routes to stimulate angiogenesis, highlighting the need for combination therapies.
- Cardiovascular health depends on VEGF’s role in blood vessel repair and maintaining vascular tone, despite the fine shades of regulation that can lead to side effects.
- Ocular disorders arise when overproduction of VEGF leads to abnormal, leaky blood vessels, necessitating targeted anti-VEGF therapies.
- Future advances are likely to focus on personalized medicine, novel gene editing strategies, and smart combination therapies that work around the intimidating challenges of the pathway.
As we continue to sort out the problematic aspects and work through the overwhelming details, the multidisciplinary efforts in research and clinical treatment will be key to unlocking the full potential of targeting VEGF signaling. This integrated approach will ultimately lead to improved quality of life for patients and a more precise way to treat both common and rare disorders associated with vascular dysfunction.
The path is complex, filled with twists and turns, but the progress made so far is a testament to the dedication of hundreds of researchers worldwide who have taken a closer look at VEGF and its many roles. By acknowledging both the simple truths and the subtle complexities, we can continue to innovate and make significant strides in the treatment of diseases that affect millions each year.
In the end, understanding and managing VEGF signaling is not just a scientific pursuit—it is a journey that promises to transform the way we approach some of the most challenging health issues of our time. With every new discovery, we are one step closer to turning the tide in diseases that once seemed overwhelmingly complicated and dangerous.
Whether it’s in the tumor microenvironment, the beating heart, or the delicate tissues of the retina, VEGF remains a cornerstone of medical research and therapeutic innovation. By embracing both its essential functions and its tangled issues, we can build a future where life is healthier and treatment options are more precise, effective, and personalized.
Originally Post From https://www.nature.com/articles/s41392-025-02249-0
Read more about this topic at
Endothelial cells decode VEGF-mediated Ca2+ signaling …
VEGF-A VEGFR-2 Signaling: Decoding the Blueprint of …